Protecting the Mental and Physical Well-Being of Frontline Healthcare Workers During COVID-19

Project Summary
Study Aims: The goal of the proposed project is to support the mental and physical well-being of U.S. healthcare workers (HCWs) to ensure high-quality patient-centered care. We propose a cluster randomized controlled trial to establish the effectiveness of a Stress First Aid (SFA) intervention compared to usual care (UC) in both hospitals, ambulatory care practices and Federally Qualified Health Centers (FQHCs) with an embedded evaluation to understand barriers and facilitators. Our specific aims are to: (1) test the comparative effectiveness of SFA versus UC on HCW mental and physical well-being; (2) understand and document any UC activities to support HCW well-being prior to implementing SFA; and (3) assess HCWs’/sites’ experiences with SFA (acceptability, likelihood of uptake, lessons learned) and impact on HCW well-being.
Background and Significance: Frontline HCWs are facing unprecedented stressors and are at risk of psychological distress and mental health disorders during COVID-19. Research from past infectious outbreaks suggests that the effects of COVID-19 on HCWs will be profound and long-lasting. Protecting HCWs’ mental and physical well-being is critical not just in terms of the direct effects, but because patients will benefit from receiving higher quality, more efficient, and patient-centered care. SFA is an evidence-informed intervention and a promising candidate to mitigate the impact of COVID-19 on HCWs’ well-being, designed as a framework of simple, supportive actions and delivered by individuals without mental health training, to mediate and mitigate impacts in atypically stressful circumstances. SFA is an excellent candidate for the proposed research, as it can be rapidly deployed, is actionable, and has great potential to be generalizable to different types of HCWs and settings. Results from this study will significantly move the field forward by providing a high quality of evidence for SFA in general and its application to HCWs during a pandemic.
Setting and Participants: This project will be conducted with two of RAND’s established partners, Clinical Directors Network, a practice-based research network (PBRN) and Vizient, a national health system. Vizient and CDN will recruit hospitals, ambulatory practices and FQHCs from which we will identify champions who will in turn train HCW teams. We will also work with Stanford Health Care, which has committed to serving as a pilot site to rapidly refine and revise the SFA training intervention in its earliest phase.
Design and Methods: Our study design is a cluster randomized controlled trial with three cohorts in approximately 40 diverse healthcare sites (12 pairs of matched hospitals and 10 pairs of ambulatory care practices/FQHCs). The 2-hour web-based intervention will be delivered to 1-4 champions in each site. The SFA training will be given by the champion to teams of HCWs to avoid disrupting important relationships and leverage existing peer support. We will roll out the intervention in three cohorts using a rapid cycle/quality improvement approach to incorporate lessons learned and stakeholder feedback in each phase into subsequent training phases. Our mixed-method evaluation approach will include qualitative interviews as well as brief online surveys pre- and post-training. For Aim 1, we will compare the effectiveness of SFA and UC on the mental and physical well-being of HCWs via a survey that captures PTSD symptoms and physical function as primary outcomes, and anxiety, depression, sleep, fatigue, social activity, burnout, and resilience as secondary outcomes. We will conduct an analysis of the comparative effectiveness analysis by applying a difference-in-differences model at follow-up. For Aims 2 and 3, we will conduct brief, structured interviews with at least one leader at SFA and UC sites (N~40) (pre- and post-intervention), and semi-structured interviews with champions (N~132) at SFA sites, and HCWs (N~66) at SFA and UC sites (post-intervention). We will conduct standardized practices for analyzing qualitative data, including systematic, team-based coding procedures. The proposed work will be shaped and informed at every stage by a highly engaged multidisciplinary working group of stakeholders (including HCWs and patients).
Results/Dissemination: The end result will be an online and printable SFA toolkit for stakeholders that will be scalable across a wide variety of settings, as well as a case study, webinar, and final report all of which will reside on a user-friendly landing page. We will share the evidence generated by this study with relevant stakeholders with the support of national organizations such as the American College of Emergency Physicians and the American Academy of Nurse Practitioners to facilitate implementation and adoption of study recommendations.

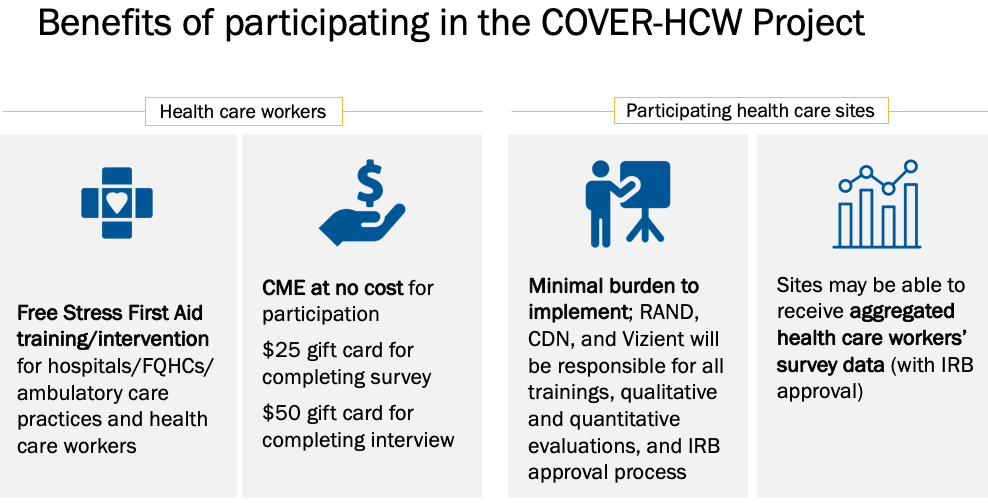
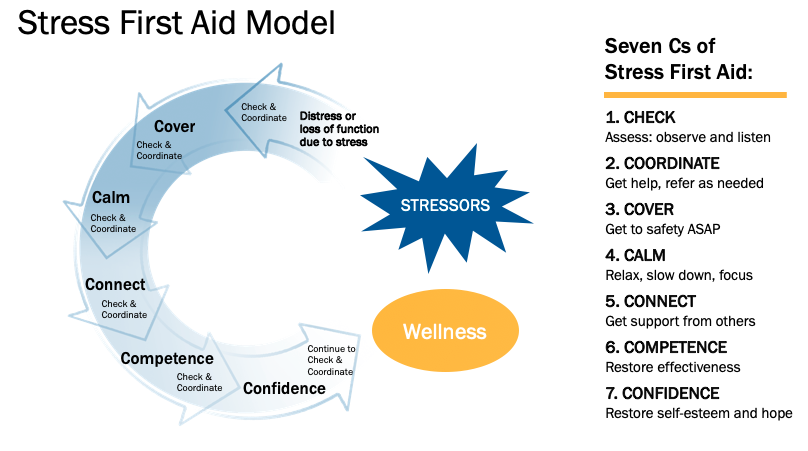
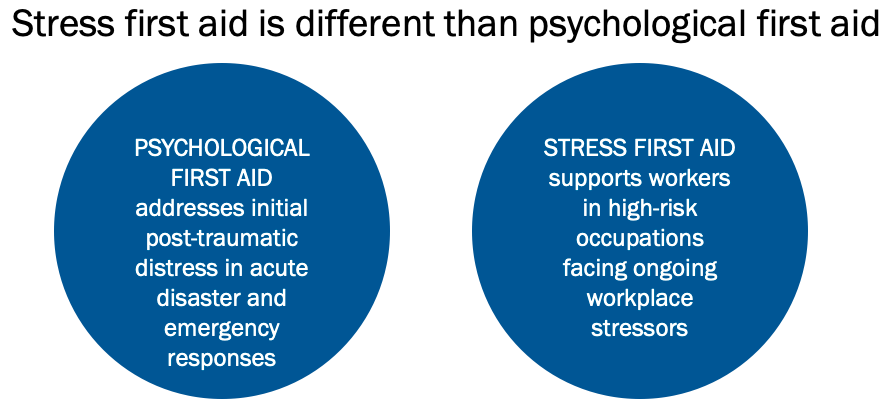
Stress First Aid in Health Care Settings
Getting Continuing Education Credit for Stress First Aid Training
Champions
Site champions attending the 2-hour training may be eligible to receive continuing education (CE) credits from an approved professional organization. We have arranged for champions with certain professional backgrounds to receive 2 CE credits for the 2-hour training.
Health Care Workers
Healthcare workers who complete the 1-hour SFA training will receive 1 CE credit.
Credit may only be obtained for completing the “live” training and passing the post-test.
Stress First Aid: When Healthcare Workers Need Care
Presenters:
Courtney Gidengil, MD, MPH | Director, Boston Office | Senior Physician Policy Researcher RAND, Boston, MA
Lisa S. Meredith, PhD | Senior Behavioral Scientist Professor, Pardee RAND Graduate School RAND, Santa Monica, CA
Rina Ramirez, MD | Physician – Chief Medical Officer, Zufall Health, Dover, NJ
Sarah Aleman, LCSW | Senior Vice President, Behavioral Health Services, Zufall Health, Dover, NJ
A live webcast brought to you by:
New York City Community Engagement Alliance to End COVID-19 Disparities (NYCEAL) – National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (NHLBI) Community Engagement Alliance Against COVID-19 Disparities (Grant No. 1OT2HL156812-01)
Protecting the Mental and Physical Well-Being of Frontline Healthcare Workers During COVID-19 (PCORI, Contract No. COVID-2020C2-10721)
The Rockefeller University Clinical and Translational Science Award Program (CTSA) (NIH-NCATS, Grant No. UL1-TR-000043)
The N2-PBRN Virtual Training Series (AHRQ, Grant No. 1P30-HS-021667)
Date/Time:
Thursday, May 16, 2024 (12:00PM-1:00PM)
Presenters:
Courtney Gidengil, MD, MPH | Senior Physician Policy Researcher, RAND Corporation | Associate Physician – Pediatrics Division of Infectious Diseases, Boston Children’s Hospital | Assistant Professor of Pediatrics, Harvard Medical School
Lisa S. Meredith, PhD | Senior Behavioral Scientist, Professor, Pardee RAND Graduate School | Research Scientist, VA HSR&D Center for the Study of Healthcare Innovation, Implementation & Policy, RAND Corporation
A live webcast brought to you by:
RAND Corporation in partnership with Clinical Directors Network, Inc. (CDN) and Vizient.
Funded by the Patient-Centered Outcomes Research Institute (PCORI) Grant #: COVID-2020C2-10721
CDN Center of Excellence (P30) for Practice-based Research and Learning,
N²: Virtual Training Series (AHRQ, Grant No. 1P30HS021667)
CME/CNE Evaluations:–
This activity is approved for 1.0 Prescribed CME/CNE credits by the American Academy of Family Physicians (AAFP).
Please click here to complete the evaluation for CME/CNE credit.
Date:
Site Champion Training Materials
Local Staff Training Materials
SFA Handouts For Staff
Extra SFA Materials from the National Center for PTSD
FAQs
Publication
Meredith, L. S., Ahluwalia, S., Chen, P. G., Dong, L., Farmer, C. M., Bouskill, K. E., Dalton, S., Qureshi, N., Blagg, T., Timmins, G., Schulson, L. B., Huilgol, S. S., Han, B., Williamson, S., Watson, P., Schnurr, P. P., Martineau, M., Davis, K., Cassells, A., Tobin, J. N., … Gidengil, C. (2024). Testing an Intervention to Improve Health Care Worker Well-Being During the COVID-19 Pandemic: A Cluster Randomized Clinical Trial. JAMA network open, 7(4), e244192. https://doi.org/10.1001/jamanetworkopen.2024.4192. PMID: 35470104
Commentary on the JAMA Publication
O’Kelly, A.C., Del Carmen, M.G., Wasfy, J.H. (2024). Learning How to Protect the Health System by Protecting the Caregivers. JAMA network open,1;7(4):e244167. doi: 10.1001/jamanetworkopen.2024.4167. PMID: 38687484
Led by the RAND Corporation in partnership with Clinical Directors Network, Inc. (CDN) and Vizient
Funded by the Patient-Centered Outcomes Research Institute (PCORI) Grant #: COVID-2020C2-10721



